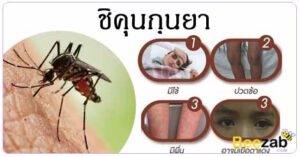
สัปดาห์แรกหลังจากติดเชื้อชิคุนกุนยาเป็นช่วงที่สำคัญที่สุดในการหลีกเลี่ยงการถูกยุงกัด เนื่องจากไวรัสจะไหลเวียนอยู่ในเลือดของผู้ติดเชื้อและสามารถส่งต่อไปยังยุงตัวใหม่ได้ สิ่งสำคัญคือต้องคลุมขาไว้และหลีกเลี่ยงไม่ให้โดนน้ำ นอกจากนี้ หากคุณมีอาการใดๆ ของโรคชิคุนกุนยา ควรติดต่อแพทย์โดยเร็วที่สุด
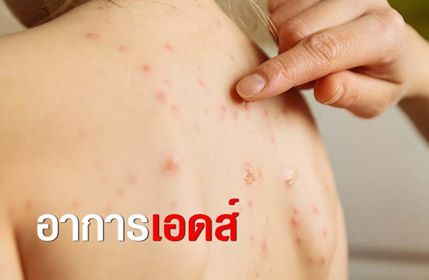
อาการที่พบบ่อยที่สุดของโรคชิคุนกุนยาคือมีไข้สูง ซึ่งอาจเกิดขึ้นได้หลายวันหรือหนึ่งสัปดาห์ อาการปวดข้อก็เป็นอาการของไวรัสเช่นกัน อาการเหล่านี้อาจส่งผลต่อร่างกายทั้งหมด แต่ส่วนใหญ่จะเกิดที่มือ เท้า และฝ่ามือ บางคนประสบปัญหาโรคหัวใจและความดันโลหิตด้วย หากคุณมีอาการเหล่านี้ ควรไปพบแพทย์ทันที
โรคชิคุนกุนยาแตกต่างจากโรคอื่นๆ ตรงที่ไม่สามารถรักษาได้จริง แต่อาการต่างๆ จะหายไปเองภายในหนึ่งสัปดาห์ สิ่งเดียวที่ทำได้คือรักษาตามอาการ ได้แก่ อาการปวดข้อและมีไข้ คุณสามารถใช้อะเซตามิโนเฟนหรือไอบูโพรเฟนเพื่อรักษาอาการปวดและเป็นไข้ได้ อย่างไรก็ตาม คุณต้องจำไว้ว่าไวรัสนี้รักษาได้ยากกว่าในผู้ที่ตั้งครรภ์ มีอายุมากกว่า หรือเป็นโรคเบาหวานหรือความดันโลหิตสูง
โรคนี้ติดต่อผ่านการถูกยุงกัด อาการมักจะปรากฏหลังจากถูกกัด 2-8 วัน แต่อาจแตกต่างกันไป อาการแรกของการติดเชื้อคือมีไข้ฉับพลันและปวดข้อ ซึ่งกินเวลาตั้งแต่หลายวันไปจนถึงหลายสัปดาห์หรือหลายเดือน ในกรณีที่รุนแรง อาการอาจเกิดขึ้นยาวนานและเป็นอันตรายถึงชีวิตได้ โชคดีที่ไม่มีวัคซีนหรือยาต้านไวรัสที่สามารถป้องกันหรือรักษาไวรัสได้ ดังนั้นการรักษาจึงเน้นที่อาการ
แม้ว่าอาการของโรคชิคุนกุนยาจะคล้ายกับโรคติดเชื้ออื่นๆ แต่ก็ไม่มีทางรักษาอาการนี้ได้ ไวรัสแพร่กระจายผ่านการถูกยุงกัด และอาจทำให้เกิดไข้และปวดข้อได้ แพทย์ของคุณจะแนะนำยาที่มีประสิทธิภาพตามอาการของคุณและปกป้องคุณจากการติดเชื้อในอนาคต ยาสำหรับการติดเชื้อ ได้แก่ อะเซตามิโนเฟนและไอบูโพรเฟน ซึ่งสามารถบรรเทาอาการปวดข้อได้
สัญญาณแรกของโรคชิคุนกุนยาคืออาการปวดข้อและมีไข้ ผู้ป่วยที่มีอาการเหล่านี้ควรปรึกษาแพทย์เพื่อรับการรักษาที่เหมาะสม ไข้ไม่ใช่ตัวบ่งชี้ที่ดีของโรคชิคุนกุนยา อาการอาจไม่รุนแรง ปานกลาง หรือรุนแรง บางคนอาจไม่มีอาการเลย แต่กรณีที่ร้ายแรงอาจกินเวลานานเป็นเดือนหรือเป็นปีก็ได้
อาการของโรคอาจจะคล้ายกับโรคอื่นๆ ผู้ที่ติดเชื้อควรติดต่อแพทย์หากพบอาการของโรค พวกเขาไม่ควรทานยาปฏิชีวนะ ในกรณีนี้คุณควรไปพบแพทย์โดยเร็วที่สุด นอกจากนี้ หากคุณมีไข้ คุณควรดื่มน้ำมากๆ เพื่อป้องกันภาวะขาดน้ำ คุณควรปรึกษาแพทย์และเยี่ยมชมเว็บไซต์ด้านสุขภาพ https://porticoonline.mx/ เพื่อดูว่าคุณเป็นโรคชิคุนกุนยาหรือไม่
สัญญาณแรกของโรคชิคุนกุนยาคืออาการปวดข้อและมีไข้ อาการปวดอาจคงอยู่เป็นเวลาหลายวันหรือหลายสัปดาห์ การเกิดโรคมักเกิดขึ้นอย่างกะทันหัน อาการปวดข้ออาจคงอยู่นานหลายเดือนหรือหลายปีก็ได้ ในกรณีที่รุนแรง ไวรัสอาจทำให้ทุพพลภาพถาวรได้ มักสังเกตเป็นเวลาหลายวันมีไข้และไม่สบายข้อ ไวรัสไม่ก่อให้เกิดโรคแทรกซ้อนร้ายแรง การติดเชื้อชิคุนกุนยาเฉียบพลันและเรื้อรังทำให้เกิดไข้และปวดข้อ
เมื่อคุณพบอาการแรกของโรคชิคุนกุนยาแล้ว คุณควรไปพบแพทย์ทันที แม้ว่าไวรัสจะไม่แพร่เชื้อจากคนสู่คน แต่ยุงกัดก็อาจแพร่เชื้อโรคถึงคุณได้ อาการของโรคชิคุนกุนยา ได้แก่ เดินก้ม ปวดข้อ มีไข้ และปวดข้ออย่างรุนแรง ผู้ที่ติดเชื้อไม่ควรละเลยอาการของโรคชิคุนกุนยา
คนส่วนใหญ่หายจากการติดเชื้อไวรัสชิคุนกุนยาภายในสองสัปดาห์ อาการปวดข้ออาจกินเวลาเป็นเดือนหรือเป็นปี แต่เมื่อไวรัสถูกกำจัดออกจากร่างกายแล้ว บุคคลนั้นก็มีแนวโน้มที่จะต้านทานการโจมตีต่อไปได้ บุคคลที่ติดเชื้อสามารถวินิจฉัยได้หลายวิธี รวมถึงการตรวจทางเซรุ่มวิทยาและการตรวจเลือด แม้ว่าอาการที่พบบ่อยที่สุดคืออาการปวดข้อ แต่ก็เป็นไปได้ที่จะมีไข้ได้ และยังสามารถแพร่เชื้อจากแม่สู่ลูกได้ด้วย